Neurosurgery Robot
May - Aug 2024
Description
This project involved the design and prototyping of a concentric tube continuum robot for combined third ventricle biopsy and ventriculostomy procedures in pediatric neurosurgery. I worked on this as a research intern at Toronto's Hospital for Sick Children (SickKids) under Dr. James Drake, focusing on mechanism design, finite element analysis, and benchtop validation.
The system leverages the inherent compliance of nested elastic tubes with pre-curved geometries to achieve dexterous tip positioning within confined anatomical spaces. By controlling the relative axial translation and rotation of concentric tube pairs, the robot can navigate complex 3D trajectories while maintaining a sub-5mm outer diameter compatible with minimally invasive neurosurgical access ports.
Clinical Motivation
Third Ventricle Pathologies
Third ventricular tumors and obstructive hydrocephalus represent challenging neurosurgical scenarios, particularly in pediatric patients. Current standard-of-care approaches include:
- Endoscopic third ventriculostomy (ETV): Creates a fenestration in the third ventricle floor to bypass CSF flow obstruction. Requires precise navigation through Foramen of Monro with limited distal articulation.
- Stereotactic biopsy: Straight-line trajectory guided by preoperative imaging. Cannot adapt to anatomical variations or avoid critical structures once inserted.
- Open craniotomy: Provides direct visualization but requires significant tissue disruption and prolonged recovery.
Unmet Clinical Need
Existing rigid endoscopes cannot simultaneously perform biopsy sampling and therapeutic fenestration in a single procedure. The concentric tube robot addresses this gap by enabling:
- Combined diagnostic and therapeutic capability: Single-pass access for tissue sampling followed by ventriculostomy
- Obstacle avoidance: Real-time trajectory adaptation to circumnavigate vessels and eloquent cortex
- Reduced patient burden: Elimination of multi-stage procedures and decreased operative time
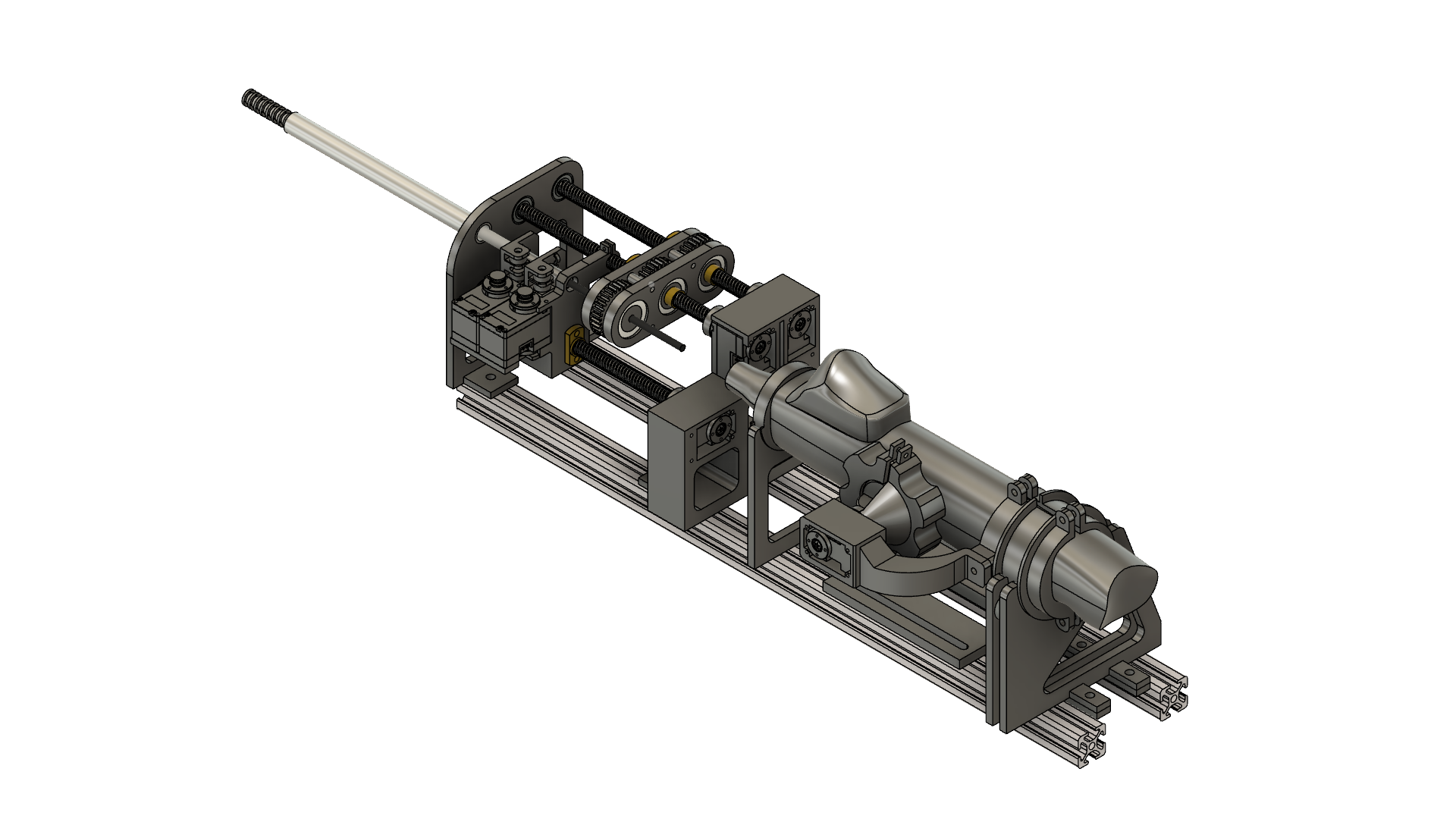
Mechanical Design
Concentric Tube Mechanics
The robot employs a three-tube nested configuration with the following specifications:
- Outer tube: Nitinol (NiTi) alloy, 3.5mm OD × 3.0mm ID, pre-curved radius 50mm, provides primary steering
- Middle tube: Nitinol 2.5mm OD × 2.0mm ID, pre-curved radius 30mm, enables secondary bending plane
- Inner tube: Stainless steel 1.5mm OD × 1.2mm ID, straight geometry, serves as biopsy instrument channel
Tube pre-curvatures are thermally set using constrained-shape annealing at 500°C for 15 minutes, followed by quenching. The superelastic properties of Nitinol (austenite finish temperature Af ≈ 10°C below body temperature) ensure shape recovery after deployment.
Actuation System
Each tube is independently actuated through a cable-driven pulley mechanism that decouples axial translation and rotation:
- Linear actuation: Lead screw (2mm pitch) driven by NEMA 17 stepper motors, providing 0.01mm positional resolution via 1/16 microstepping
- Rotational actuation: Timing belt transmission (1:2 reduction) connected to tube-mounted pulleys, enabling ±180° rotation with 0.9° resolution
- Encoder feedback: Magnetic rotary encoders (12-bit, 0.088° resolution) mounted on each motor shaft for closed-loop position control
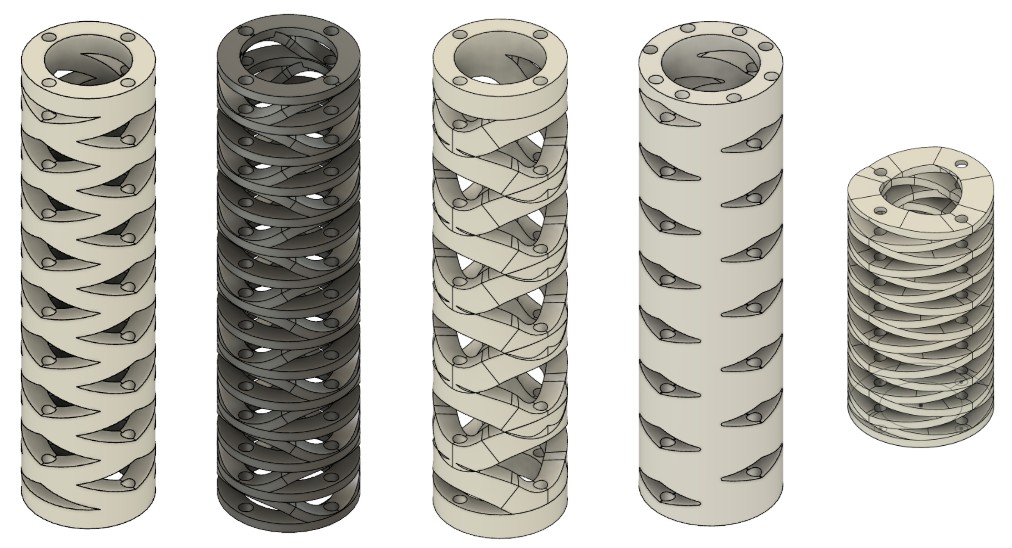
Patterned Tube Innovation
Motivation for Patterning
Standard solid-wall Nitinol tubes exhibit limited curvature variability once manufactured. To enable adaptive stiffness profiles and enhanced compliance, I designed laser-cut patterned tubes with spatially varying bending stiffness.
Pattern Design
The pattern consists of helical cuts arranged in a periodic lattice structure:
- Cut geometry: 0.3mm kerf width, 60° helix angle, 5mm pitch
- Material removal ratio: 40% of tube wall in high-flexibility regions, tapering to 10% in proximal sections
- Fabrication: Fiber laser micromachining (1064 nm wavelength, 20 W average power, 50 kHz pulse rate)
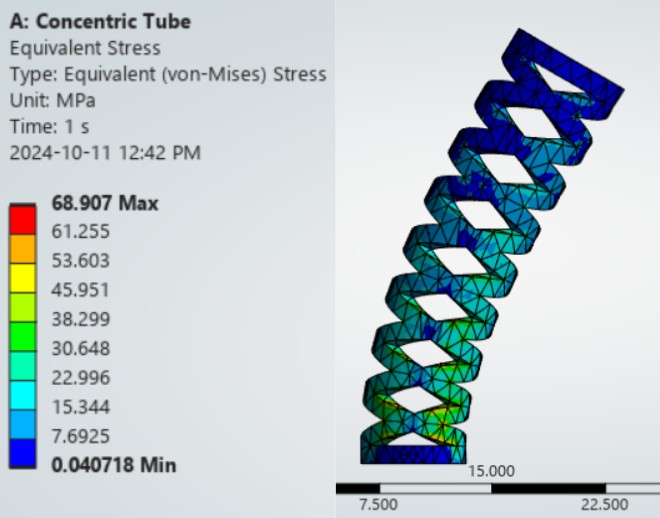
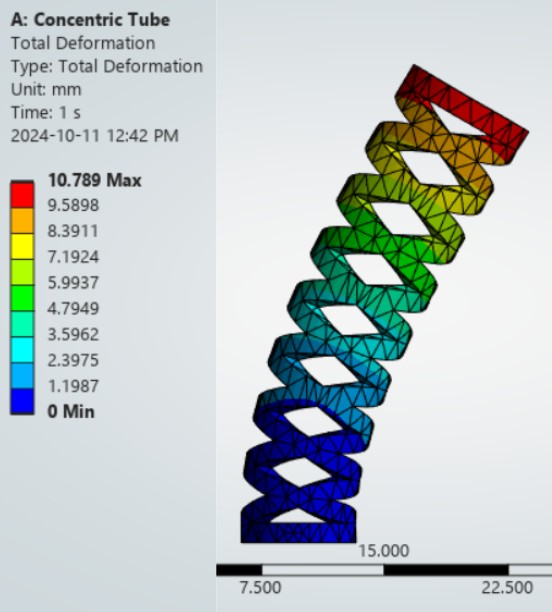
Finite Element Analysis
Modeling Objectives
FEA simulations were conducted to predict:
- Bending stiffness: Moment-curvature relationship for each tube configuration
- Stress concentration: Peak von Mises stress in patterned regions under physiological loading
- Failure prediction: Safety factors against yield (Nitinol: 195-690 MPa depending on phase) and fatigue (10^7 cycle endurance limit)
- Elastic shape deployment: Predicted deployed curvature as a function of tube extension and relative rotation
Simulation Parameters
- Solver: ANSYS Mechanical APDL, nonlinear static structural analysis
- Material model: Superelastic Nitinol with stress-induced martensitic transformation (austenite E = 75 GPa, martensite E = 28 GPa)
- Mesh: Hexahedral elements (SOLID186), 0.1mm element size in cut regions, adaptive refinement at stress singularities
- Boundary conditions: Cantilevered proximal end, prescribed displacement at distal tip to simulate bending
Surgical Tool Integration
Borescope Handle Design
To enable visual feedback during robot deployment, I designed a custom handle interface for integrating a medical-grade borescope (1.8mm diameter, 0° viewing angle) into the concentric tube assembly.
- Ergonomic handle: 3D-printed in biocompatible resin (Formlabs Surgical Guide), autoclavable geometry
- Sealed optics channel: O-ring sealed interface (Buna-N, -40 to 120°C) to prevent CSF ingress
- Working channel integration: 1.2mm lumen for irrigation, suction, or biopsy forceps deployment
- Fiber optic coupling: Standard SMA connector for external LED light source (5000K, 150 lumen output)

Benchtop Validation & Results
Trajectory Accuracy Testing
Workspace characterization performed in transparent silicone brain phantom:
- Reachable workspace: 45mm × 35mm × 30mm ellipsoidal volume centered at Foramen of Monro entry point
- Tip positioning accuracy: Mean error 2.3 ± 0.8 mm across 50 target points (measured via stereoscopic camera tracking)
- Deployment time: 45-60 seconds from cortical entry to third ventricle floor fenestration point
Patterned Tube Performance
- Bending stiffness reduction: 65% decrease in effective EI (flexural rigidity) compared to solid tube of equivalent outer diameter
- Stress mitigation: Peak von Mises stress 340 MPa (safety factor 1.8 against Nitinol yield), validated by experimental strain gauge measurements
- Fatigue endurance: >1000 full-extension cycles without observable crack propagation (SEM microscopy confirmation)
Future Development & Translation
Technical Enhancements
- Force sensing: Integrate fiber Bragg grating (FBG) strain sensors into tube walls for contact force estimation and collision avoidance
- Shape sensing: Multi-core fiber optic shape reconstruction (0.5mm resolution) for real-time 3D visualization overlaid on preoperative MRI
- MRI compatibility: Replace stepper motors with piezoelectric ultrasonic actuators to enable intraoperative imaging guidance
Clinical Translation Path
- Cadaver validation studies in collaboration with SickKids surgical simulation lab
- FDA 510(k) regulatory pathway assessment (probable Class II medical device classification)
- GLP toxicology testing of Nitinol and resin components per ISO 10993 biocompatibility standards
- Design for Manufacturing (DFM) analysis to reduce per-unit cost from $8000 (prototype) to <$2000 (manufacturing scale)